 |
||
 |
||
|
|
|
Active Hydrogen
Adrenal Extracts
Alanine
Alpha-Linolenic Acid
Alpha-Lipoic Acid
AMP
Amylase Inhibitors
Arginine
Bee Pollen
Beta Carotene
Beta-glucan
Betaine
Beta-Sitosterol
Biotin
Borage Oil
Boron
Bovine Cartilage
Bovine Colostrum
Brewer's Yeast
Bromelain
Calcium
Capsaicin
Carnitine
Carnosine
Chitosan
Chloride
Chlorophyll
Chondroitin
Chromium
CLA
Cobalt
Coenzyme Q10
Copper
Creatine
Cysteine
DHA
DHEA
DMAE
EGCG
Evening Primrose Oil
5-HTP
Fiber (Insoluble)
Fiber (Soluble)
Fish Oil
Flavonoids
Fluoride
Folate
Fumaric Acid
GABA
Gamma-Linolenic Acid
Glucomannan
Glucosamine
Glutamic Acid
Glutamine
Glutathione
Glycine
Grape Seed Extract
Histidine
HMB
Hydroxycitric Acid
Indole
Inosine
Inositol
Iodine
Ipriflavone
Iron
Isoleucine
Lactase
Lecithin
Leucine
Lipase
Lutein
Lycopene
Lysine
Magnesium
Malic Acid
Manganese
Mannose
Melatonin
Methionine
Methoxyisoflavone
Molybdenum
MSM
N-Acetyl Cysteine
NADH
Naringin
Niacin
Octacosanol
Oligosaccharides
Olive Leaf Extract
Ornithine
Oryzanol
PABA
Pancreatic Enzymes
Pantothenic Acid
Phenylalanine
Phosphatidylserine
Phosphorus
Phytic Acid
Policosanol
Potassium
Pregnenolone
Probiotics
Propolis
Psyllium
Pyridoxine
Pyruvate
Quercetin
Resveratrol
Retinol
Riboflavin
Ribose
Royal Jelly
SAMe
Selenium
Shark Cartilage
Silicon
Sodium
Spirulina
Spleen Extracts
St. John's Wort
Strontium
Sulforaphane
Sulfur
Taurine
Thiamine
Tocopherol
Tea Tree Oil
Tyrosine
Usnic Acid
Valine
Vanadium
Vinpocetine
Vitamin A
Vitamin B1
Vitamin B2
Vitamin B3
Vitamin B5
Vitamin B6
Vitamin B9
Vitamin B12
Vitamin C
Vitamin D
Vitamin H
Vitamin K
Whey Protein
Xylitol
Zinc
Abalone Shell (shi jue ming)
Abutilon Seed (dong kui zi) Acanthopanax Bark (wu jia pi) Achyranthes (niu xi) Aconite (fu zi) Acorus (shi chang pu) Adenophora Root (nan sha shen) Agkistrodon (bai hua she) Agrimony (xian he cao) Ailanthus Bark (chun pi) Akebia Fruit (ba yue zha) Albizzia Bark (he huan pi) Albizzia Flower (he huan hua) Alfalfa (medicago sativa) Alisma (ze xie) Aloe (lu hui) Alum (bai fan) Amber (hu po) Ampelopsis (bai lian) Andrographis (chuan xin lian) Anemarrhena (zhi mu) Antelope's Horn (ling yang jiao) Apricot Seed (xing ren) Areca Peel (da fu pi) Areca Seed (bing lang) Arisaema (tian nan xing) Ark Shell (wa leng zi) Arnebia (zi cao or ying zi cao) Arnica (arnica montana) Artichoke Leaves (Cynara scolymus) Ash bark (qin pi) Ashwagandha (withania somniferum) Aster (zi wan) Astragalus (huang qi) Aurantium (zhi ke [qiao]) Bamboo Juice (zhu li) Bamboo Shavings (zhu ru) Belamcanda Rhizome (she gan) Benincasa Peel (dong gua pi) Benincasa Seed (dong gua xi/ren) Benzoin (an xi xiang) Bilberry (yue ju) Biota Leaf (ce bai ye) Biota Seed (bai zi ren) Bitter Melon (ku gua) Bitter Orange Peel (ju hong) Black Cohosh (sheng ma) Black Plum (wu mei) Black Sesame Seed (hei zhi ma) Bletilla (bai ji) Boneset (ze lan) Borax (peng sha) Borneol (bing pian) Bottle Brush (mu zei) Buddleia (mi meng hua) Buffalo Horn (shui niu jiao) Bulrush (pu huang) Bupleurum (chai hu) Burdock (niu bang zi) Camphor (zhang nao) Capillaris (yin chen hao) Cardamon Seed (sha ren) Carpesium (he shi) Cassia Seed (jue ming zi) Catechu (er cha) Cat's Claw (uncaria tomentosa) Cephalanoplos (xiao ji) Celosia Seed (qing xiang zi) Centipede (wu gong) Chaenomeles Fruit(mu gua) Chalcanthite (dan fan) Chebula Fruit (he zi) Chinese Gall (wu bei zi) Chinese Raspberry (fu pen zi) Chrysanthemum (ju hua) Cibotium (gou ji) Cinnabar (zhu sha) Cinnamon (rou gui or gui zhi) Cistanche (rou cong rong) Citron (xiang yuan) Citrus Peel (chen pi) Clam Shell (hai ge ke/qiao) Clematis (wei ling xian) Cloves (ding xiang) Cnidium Seed (she chuang zi) Codonopsis (dang shen) Coix Seed (yi yi ren) Coptis (huang lian) Cordyceps (dong chong) Coriander (hu sui) Corn Silk (yu mi xu) Cornus (shan zhu yu) Corydalis (yan hu suo) Costus (mu xiang) Cranberry (vaccinium macrocarpon) Cremastra (shan ci gu) Croton Seed (ba dou) Curculigo (xian mao) Cuscuta (tu si zi) Cuttlefish Bone (hai piao xiao) Cymbopogon (xiang mao) Cynanchum (bai qian) Cynomorium (suo yang) Cyperus (xiang fu) Dalbergia (jiang xiang) Damiana (turnera diffusa) Dandelion (pu gong ying) Deer Antler (lu rong) Dendrobium (shi hu) Devil's Claw (harpagophytum procumbens) Dianthus (qu mai) Dichroa Root (chang shan) Dittany Bark (bai xian pi) Dong Quai (tang kuei) Dragon Bone (long gu) Dragon's Blood (xue jie) Drynaria (gu sui bu) Dryopteris (guan zhong) Earthworm (di long) Eclipta (han lian cao) Elder (sambucus nigra or sambucus canadensis) Elsholtzia (xiang ru) Ephedra (ma huang) Epimedium (yin yang huo) Erythrina Bark (hai tong pi) Eucalyptus (eucalyptus globulus) Eucommia Bark (du zhong) Eupatorium (pei lan) Euphorbia Root (gan sui or kan sui) Euryale Seed (qian shi) Evodia (wu zhu yu) Fennel (xiao hui xiang) Fenugreek (hu lu ba) Fermented Soybeans (dan dou chi) Flaxseed (ya ma zi) Fo Ti (he shou wu) Forsythia (lian qiao) Frankincense (ru xiang) Fritillaria (chuan bei mu) Gadfly (meng chong) Galanga (gao liang jiang) Galena (mi tuo seng) Gambir (gou teng) Gardenia (zhi zi) Garlic (da suan) Gastrodia (tian ma) Gecko (ge jie) Gelatin (e jiao) Genkwa (yuan hua) Germinated Barley (mai ya) Ginger (gan [sheng] jiang) Ginkgo Biloba (yin xing yi) Ginseng, American (xi yang shen) Ginseng, Asian (dong yang shen) Ginseng, Siberian (wu jia shen) Glehnia (sha shen) Glorybower (chou wu tong) Goldenseal (bai mao liang) Gotu Kola (luei gong gen) Green Tea (lu cha) Gymnema (gymnema sylvestre) Gynostemma (jiao gu lan) Gypsum (shi gao) Halloysite (chi shi zhi) Hawthorn (shan zha) Hemp Seed (huo ma ren) Homalomena (qian nian jian) Honey (feng mi) Honeysuckle Flower (jin yin hua) Honeysuckle Stem (ren dong teng) Houttuynia (yu xing cao) Huperzia (qian ceng ta) Hyacinth Bean (bai bian dou) Hyssop (huo xiang) Ilex (mao dong qing) Imperata (bai mao gen) Indigo (qing dai) Inula (xuan fu hua) Isatis Leaf (da qing ye) Isatis Root (ban lan gen) Java Brucea (ya dan zi) Jujube (da zao) Juncus (deng xin cao) Kadsura Stem (hai feng teng) Katsumadai Seed (cao dou kou) Kelp (kun bu) Knotweed (bian xu) Knoxia root (hong da ji) Kochia (di fu zi) Lapis (meng shi) Leech (shui zhi) Leechee Nut (li zhi he) Leonorus (yi mu cao) Lepidium Seed (ting li zi) Licorice (gan cao) Ligusticum (chuan xiong) Ligustrum (nč zhen zi) Lily Bulb (bai he) Limonite (yu liang shi) Lindera (wu yao) Litsea (bi cheng qie) Lobelia (ban bian lian) Longan (long yan hua [rou]) Lophatherum (dan zhu ye) Loquat Leaf (pi pa ye) Lotus Leaf (he ye) Lotus Node (ou jie) Lotus Seed (lian zi) Lotus Stamen (lian xu) Luffa (si gua luo) Lycium Bark (di gu pi) Lycium Fruit (gou qi zi) Lygodium (hai jin sha) Lysimachia (jin qian cao) Magnetite (ci shi) Magnolia Bark (hou po) Magnolia Flower (xin yi hua) Maitake (grifola frondosa) Marigold (c. officinalis) Massa Fermentata (shen qu) Milk Thistle (silybum marianum) Millettia (ji xue teng) Mint (bo he) Mirabilite (mang xiao) Morinda Root (ba ji tian) Mugwort Leaf (ai ye) Mulberry Bark (sang bai pi) Mulberry Leaf (sang ye) Mulberry Twig (sang zhi) Mullein (jia yan ye) Musk (she xiang) Myrrh (mo yao) Notoginseng (san qi) Notopterygium (qiang huo) Nutmeg (rou dou kou) Oldenlandia (bai hua she she cao) Omphalia (lei wan) Onion (yang cong) Ophicalcite (hua rui shi) Ophiopogon (mai dong) Oroxylum Seed (mu hu die) Oryza (gu ya) Oyster Shell (mu li) Passion Flower (passiflora incarnata) Patrinia (bai jiang cao) Pau D'Arco (tabebuia avellanedae) Peach Seed (tao ren) Pearl (zhen zhu [mu]) Perilla Leaf (su ye) Perilla Seed (su zi) Perilla Stem (su geng) Persimmon (shi di) Pharbitis Seed (qian niu zi) Phaseolus (chi xiao dou) Phellodendron (huang bai) Phragmites (lu gen) Picrorhiza (hu huang lian) Pinellia (ban xia) Pine Knots (song jie) Pipe Fish (hai long) Plantain Seed (che qian zi) Platycodon (jie geng) Polygala (yuan zhi) Polygonatum (huang jing) Polyporus (zhu ling) Poppy Capsule (ying su qiao) Poria (fu ling) Prickly Ash Peel (hua jiao) Prinsepia Seed (rui ren/zi) Prunella (xia ku cao) Prunus Seed (yu li ren) Pseudostellaria (tai zi shen) Psoralea (bu gu zhi) Pueraria (ge gen) Pulsatilla (bai tou weng) Pumice (fu hai shi) Pumpkin Seed (nan gua zi) Purslane (ma chi xian) Pyrite (zi ran tong) Pyrrosia Leaf (shi wei) Quisqualis (shi jun zi) Radish (lai fu zi) Realgar (xiong huang) Red Atractylodes (cang zhu) Red Clover (trifolium pratense) Red Ochre (dai zhe shi) Red Peony (chi shao) Red Sage Root (dan shen) Rehmannia (shu di huang) Reishi (ling zhi) Rhubarb (da huang) Rice Paper Pith (tong cao) Rose (mei gui hua) Rosemary (mi die xiang) Safflower (hong hua) Saffron (fan hong hua) Sandalwood (tan xiang) Sanguisorba Root (di yu) Sappan Wood (su mu) Sargent Gloryvine (hong teng) Saw Palmetto (ju zong lu) Schefflera (qi ye lian) Schisandra (wu wei zi) Schizonepeta (jing jie) Scirpus (san leng) Scopolia (S. carniolica Jacq.) Scorpion (quan xie) Scrophularia (xuan shen) Scutellaria (huang qin) Sea Cucumber (hai shen) Sea Horse (hai ma) Seaweed (hai zao) Selaginella (shi shang bai) Senna (fan xie ye) Shiitake (hua gu) Siegesbeckia (xi xian cao) Siler Root (fang feng) Slippery Elm (ulmus fulva) Smilax (tu fu ling) Smithsonite (lu gan shi) Sophora Flower (huai hua mi) Sophora Root (ku shen) Spirodela (fu ping) Stellaria (yin chai hu) Stemona (bai bu) Stephania (fang ji [han]) Sweet Annie (qing hao) Teasel Root (xu duan) Tiger Bone (hu gu) Torreya Seed (fei zi) Tortoise Plastron (gui ban) Tremella (bai mu er) Trichosanthes Fruit (gua lou) Trichosanthes Root (tian hua fen) Trichosanthes Seed (gua lou ren) Tsaoko Fruit (cao guo) Turmeric (jiang huang) Turtle Shell (bie jia) Tussilago (kuan dong hua) Urtica (xun ma) Uva ursi (arctostaphylos uva-ursi) Vaccaria Seed (wang bu lui xing) Valerian (jie cao) Veratrum (li lu) Viola (zi hua di ding) Vitex (man jing zi) Walnut (hu tao ren) Watermelon (xi gua) White Atractylodes (bai zhu) White Mustard Seed (bai jie ze) White Peony (bai shao) Wild Asparagus (tian men dong) Windmill Palm (zong lu pi/tan) Xanthium (cang er zi) Zedoary (e zhu) |
Gut Health and Good Health
A Role For Pre- and Probiotics?
By Campbell Berry-Kilgour, BSc (Hons) In a Persian translation of the Old Testament (Gen. 18:8) it states that “Abraham owed his longevity to the consumption of sour milk.” In 76 B.C., the Roman historian Plinius recommended the administration of fermented milk products for treating gastroenteritis.1 The long history of health claims concerning living microorganisms in food, particularly lactic acid bacteria, may be about to assume renewed importance, especially as digestive ailments continue to plague our modern lifestyle.
A number of factors can change the balance of intestinal microflora in favor of harmful bacteria. These include antibiotic therapy, emotional stress, poor diet and aging. Two classes of natural products, pre- and probiotics, have been shown to be valuable agents that work to optimize gut microflora, and in so doing offer hope in terms of intestinal wellness, lowering the incidence and severity of such aforementioned problems. Intestinal Balance The primary role of the human gastrointestinal tract is to digest and absorb nutrients from food. Additionally, the gut mucosa represents the first line of defence against bacterial toxins and infections caused by bacteria, viruses or parasites. In fact, the intestine is the largest immune organ inside our body, where it hosts billions of diverse species of micro-organisms that inhabit our alimentary canal. In the broadest context, these organisms can be classified as either beneficial or pathogenic. Importantly, it is now recognized that optimal health requires a balance of beneficial bacteria over their pathogenic counterparts. A number of factors can change the balance of intestinal microflora in favor of harmful bacteria. These include antibiotic therapy, emotional stress, poor diet and aging.3 In addition to disease states, the mucosa of the alimentary canal is the target of several disturbances induced by modern lifestyle and characteristically Western eating habits.4 Moreover, intervention with products aimed at increasing the numbers of beneficial bacteria have been shown to help combat antibiotic-induced diarrhea, irritable bowel syndrome, eczema, ulcerative colitis; reduce the incidence of vaginal yeast infections; and exert positive effects on the immune system. Prebiotics Less well-known than their probiotic cousins, prebiotics have been shown however to convey positive health benefits both independently, and in collaboration with probiotics. The official definition of a prebiotic is a “nondigestible food ingredient(s) that beneficially affects the host by selectively stimulating the growth and activity of one species or a limited number of species of bacteria in the colon.”5 The various oligosaccharides classified as prebiotics include fructo-oligosacchardies (FOS), inulins, isomalto-oligosaccharides, lactulose, oligofructose, soy-oligosaccharides and xylo-oligosaccharides. More recently, the prebiotic properties of New Zealand beech honey (honeydew honey oligosaccharides, HDO) have been described.6 HDO has strong antipathogenic activity against E.coli and Salmonella enteritidis, while having an effect similar to that of FOS. Prebiotics may encourage anticarcinogenic, antimicrobial, hypolipidaemic and glucose-modulating activities. Primarily, these short-chain polysaccharides serve as a “food supply” for beneficial bacteria resident in the large bowel. Prebiotics are principally oligosaccharides that act by stimulating the growth of bifidobacteria, and they have been shown to prevent the symptoms associated with irritable bowel disease, improve bowel function, resulting in increased stool frequency and weight. Prebiotics support healthy digestive function by preventing intestinal attachment of pathogens by acting as receptor homologues. An important feature of prebiotics is their potential benefit in terms of risk reduction of colorectal cancer. While the possible anti-carcinogenic activity of prebiotics is not well understood, it may be accounted for, in part at least, by the possible actions of butyrate. Butyrate is a breakdown product produced by bacterial fermentation of prebiotic oligosaccharides in the colon. Some studies suggest that butyrate acts to induce growth arrest and cell differentiation and upregulate apoptosis. It has been reported that “enzyme induction butyrate, or by the microflora and increased activity by prebiotics, may be an important mechanism or protection against carcinogen-enhanced colon cancer.”7 Prebiotic oligosaccharides have also been shown to reduce the incidence of atopic dermatitis in infants.8 The possible antimicrobial effect of prebiotics can be accounted for by their ability to increase the proliferation of lactobacilli and bifidobacteria. These bacteria act directly to strengthen the intestinal mucosa, thus preventing the attachment of pathogenic bacteria. These beneficial bacteria may also stimulate antigen-specific and nonspecific immune responses. Prebiotics have been shown to produce favorable lipid effects. While the exact mechanisms are poorly understood, it has been suggested that propionate, another fermentation product produced from the breakdown of prebiotic in the colon, acts to inhibit the enzyme HMG-CoA reductase, limiting cholesterol synthesis.9 In terms of reported adverse reactions, prebiotic oligosaccharides given in doses up to 10 grams daily are usually well-tolerated. Higher doses may cause gastrointestinal upset such as flatulence, bloating and diarrhea. Any individuals undergoing whole-body irradiation or radiation to the gastrointestinal tract should not be given prebiotic supplements. Concomitant use of calcium or magnesium with a prebiotic will increase the colonic absorption of these minerals. Concomitant administration of pre- and probiotics may increase the effectiveness of both agents – this is termed synbiosis. Probiotics Food products fermented by lactic acid bacteria have long been used for their proposed health-promoting properties. The probiotic concept was born in the early 20th century by the Russian scientist Élie Metchnikoff, who was awarded the Nobel Prize in 1908. Dr. Metchnikoff proposed that the health, well-being, and longevity of Balkan populations were attributable to their consumption of large quantities of fermented milks containing beneficial microorganisms.10 A century after this pioneering work, the probiotic concept has gained significant credibility; as much progress has been made in demonstrating probiotic health benefits. Today, probiotics are defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host”. Lactobacillus acidophilus and Bifidobacterium lactis are two of the bacterial species for which probiotic attributes have been proven. There are however many products available commercially, either as single strains or as combinations. Because probiotics do not permanently colonize the intestine, they must be taken in sufficient quantities (>1 x 1010/d) to maintain adequate amounts in the colon. In addition, they must be of human origin and be able to adhere to intestinal mucosa. By positively influencing intestinal microflora, probiotics have been shown to reduce the incidence and risks associated with many gastrointestinal disorders, including antibiotic-induced diarrhea, symptoms associated with irritable bowel syndrome, and Candida overgrowth. In addition, probiotics appear to boost immune function, and have been found to increase absorption of key nutrients, including calcium, copper, magnesium and iron. In the upper digestive tract, probiotics may be utilized to balance bacteria levels to combat gut-caused halitosis. Balanced Intestinal MicrofloraThe complex ecosystem of the adult intestinal microflora comprises approximately 500 different species of bacteria. The two major strains of beneficial bacteria are lactobacilli and bifidobacteria. The probiotic Bifidobacterium bifidum is among the most prominent bacteria in the intestinal microflora. It creates a healthy environment for the production of B-complex vitamins and vitamin K. In babies, almost all gut bacteria are Bifidobacterium; levels slowly decline after weaning, through adolescence and adult life, and decline more rapidly in old age. Pre- and probiotics offer the health practitioner useful agents to positively influence intestinal microflora. The use of antibiotics can cause havoc to and alter our normal intestinal flora. This results in an increase in the production of ammonia, which in turn causes irritation to the intestinal mucosa. Because probiotic such as Bifidobacteria aid digestion, any decrease in functional populations often leads to digestive disorders. Most importantly, intestinal microflora may be maintained, or restored to normal, from an unbalanced state, by introduction of suitable strains of probiotic. There is currently great interest in the potential for probiotics to promote immune development, and also as a supplementary treatment for a broad range of infectious and inflammatory diseases.11 The greatest potential for benefit appears to be in the areas of infections or inflammation of the gastrointestinal tract, and for the prevention of allergic disorders. Probiotics have also been shown to inhibit the growth of Candida albicans in the digestive tract. A clinical study in patients infected with Candida showed that 89 percent of patients in the treatment group (n=36) had lower symptom scores after treatment with a supplement containing Lactobacillus acidophilus and Bifidobacterium bifidum. The value of prophylactic use of suitable strains of probiotic has also been shown through well-designed clinical trials, with lactic acid bacteria being shown to reduce the incidence of traveller’s diarrhea.12 Similar results have been shown in the prevention of antibiotic-associated diarrhea.13 Other complaints also respond favourably to intervention with probiotics. For example, there is an increasing body of evidence showing that probiotics help reduce eczema in allergic infants. Researchers from the University of Helsinki demonstrated that supplementation with Lactobacillus GG resulted in a 65 percent reduction in allergy symptoms.14 Viability Is Critical for Efficacy A number of issues impact the usefulness of probiotics. The strains used must be able to survive their journey through the acid environment of the stomach, and then the alkaline duodenum. In addition, to be effective, probiotics must adhere to the intestinal mucosa. It is well known that probiotics are far more fragile than prebiotics, and will degrade when exposed to heat, oxygen, and moisture. A 2005 Consumer Report15 issued a summary showing that many commercially produced probiotic supplements failed to meet their label claims, with fewer than 50 percent containing their claimed number of active bacteria when tested. In order to ensure viability, the manufacturing process, packaging and strain selection are critical. For the health professional, or indeed consumer, there is clearly no benefit in using a product that contains little or no live bacteria. Practitioners should actively seek out those products that are available with label claims declaring guaranteed counts to their expiry date, usually when the product is stored as directed. This provides greater reassurance of activity than those products that merely declare a count at “time of manufacture.” Pre- and probiotics offer the health practitioner useful agents to positively influence intestinal microflora. These naturally occurring food ingredients and beneficial microorganisms offer exciting opportunities for the prevention and treatment of a wide variety of conditions associated with impaired intestinal mucosa and chronic inflammatory responses. References
|
Nutritional Wellness News Update:

Other Alternative Health Sites
Toyour Health
ChiroWeb
ChiroFind
Dynamic Chiropractic
DC Practice Insights
Acupuncture Today
Massage Today
Naturopathy Digest
Chiropractic Research Review
Spa Therapy
|


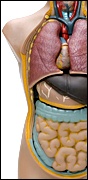 Evidence abounds supporting the fact that our genes tolerate poorly the dramatic changes in lifestyle that have occurred, especially in eating habits, during the past 100 years.2 In 2003, more than 52 million Americans sought medical treatment for digestive problems. The typical 21st century Western diet – highly processed, low in fiber, high in fat – is much to blame. This picture is further compounded by inappropriate antibiotic use and lifestyle factors such as stress, obesity and lack of exercise. Allergic disease, atopic eczema, allergic rhinitis and asthma, together with chronic inflammatory bowel disease, Crohn’s disease, ulcerative colitis and arthritis all represent chronic conditions rising in incidence in the Western world. These diseases are usually associated with impairment of gut-barrier function – suggestive of the fact that with the passing of time, the effectiveness of our innate host defense mechanism, mediated by the gut, has decreased.
Evidence abounds supporting the fact that our genes tolerate poorly the dramatic changes in lifestyle that have occurred, especially in eating habits, during the past 100 years.2 In 2003, more than 52 million Americans sought medical treatment for digestive problems. The typical 21st century Western diet – highly processed, low in fiber, high in fat – is much to blame. This picture is further compounded by inappropriate antibiotic use and lifestyle factors such as stress, obesity and lack of exercise. Allergic disease, atopic eczema, allergic rhinitis and asthma, together with chronic inflammatory bowel disease, Crohn’s disease, ulcerative colitis and arthritis all represent chronic conditions rising in incidence in the Western world. These diseases are usually associated with impairment of gut-barrier function – suggestive of the fact that with the passing of time, the effectiveness of our innate host defense mechanism, mediated by the gut, has decreased.